In the last decades, light microscopy has emerged as an indispensable tool in the life sciences and in the materials sciences. Its strengths lie in its ability to get a three-dimensional image from the interior of sufficiently translucent samples. Conventional light microscopy suffers from the drawback that its resolution is limited to 200 – 300 nm due to the wave nature of light. Therefore, crucial dimensions like those on the molecular scale stayed out of reach.
Super resolution fluorescence microscopy avoids this limitation by using a trick. The properties of fluorescent markers are used such that the information within a blurred spot is read out sequentially.
In the life sciences, optical nanoscopy helps to visualize structure and function in living cells. This provides fantastic possibilities to gain insight into the ongoing molecular processes.
This knowledge is one of the keys to the understanding of diseases and the development of new diagnostic tools, novel drugs and their therapeutic use.
Fields of application in the materials sciences are the simulation of atomic and molecular systems with the help of colloidal particles or the investigation of the self-organization of nanostructures. The size of the used colloidal particles is not limited by the diffraction limit and can therefore be used as an important control parameter. Moreover, the basic principles of optical nanoscopy can not only be applied for imaging, but also for sample manipulation, e. g. by lithography of small structures below the diffraction limit for the fabrication of computer chips.
The “Optical Nanoscopy” department, established in the third quarter of 2010, performs applied research in the field of “fluorescence microscopy below the diffraction limit” and develops new methods. Its emphasis lies on two complementary far-field high resolution techniques in fluorescence microscopy:
- STED microscopy
- SMS microscopy
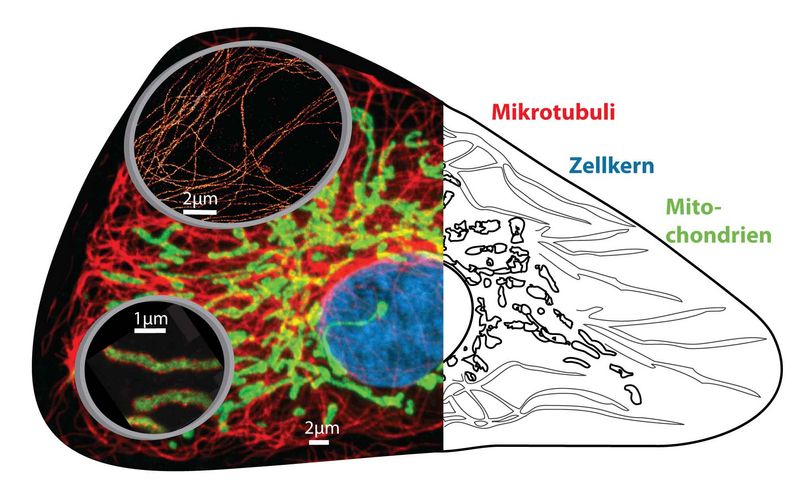
Collage of optical nanoscopy in cells. Conventional diffraction-limited fluorescence microscopy image (left, background) and schematic view (right) of cell organelles. Magnifying glasses (left, foreground) show nanoscopic images of microtubules (top) and mitochondria (bottom) with resolution of only some 10 nanometers
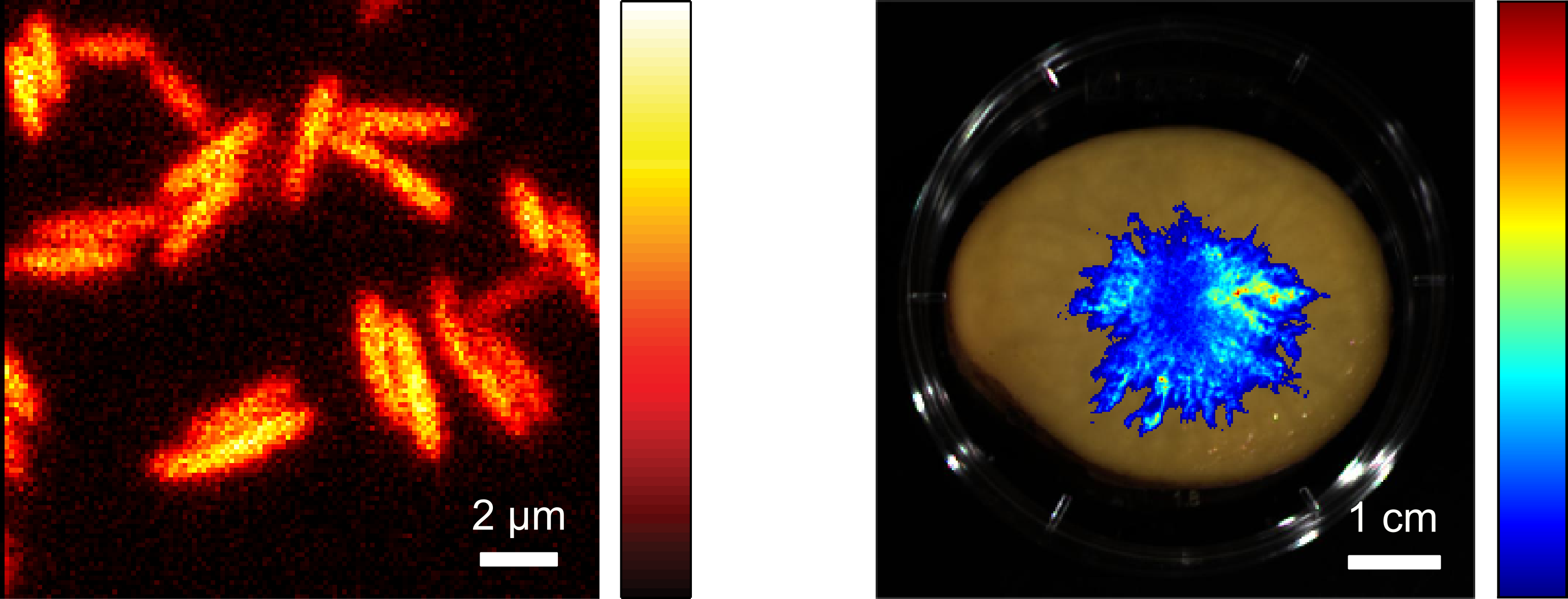
Another research focus of the department is the imaging of living cells using bioluminescence. In this process, which can be found in nature for example in fireflies, light is generated by the cells themselves through a biochemical reaction and can be used directly for imaging. Unlike with fluorescence-based methods, no excitation light is required, and hence imaging can be carried out gently over long periods of time without affecting the marker molecules and cells by external light.
Of particular importance is bioluminescence imaging without the addition of chemical substrates that are usually required to generate light. For this purpose, we use the bacterial bioluminescence system which can be applied not only in bacteria but also in other cell types such as human cells. The intensity of the bioluminescence light provides additional information about cell vitality and can therefore be used to investigate the effects of cell-damaging substances or certain drugs.
Various measuring systems are being developed and used for bioluminescence imaging on different size scales. These include camera- and fiber-based systems for recording macroscopic samples and a microscope for imaging individual cells.
STED microscopy
The key to overcome the diffraction barrier with freely propagating light waves is to use the properties of the fluorescent markers such that they are transferred between a bright, detectable state and a dark, non-detectable state or vice versa.
This is done in such a way that the region in which the markers can exist in their bright state is spatially confined.
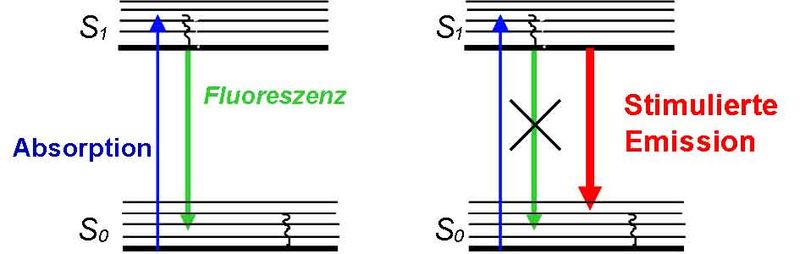
The STED (STimulated Emission Depletion) principle uses stimulated emission to “switch” the fluorescent markers from their (bright) excited electronic state S1 to the (dark) electronic ground state S0.
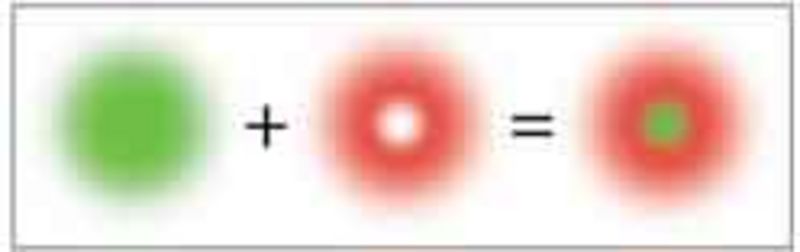
In order to confine the region in which the markers are allowed to fluoresce beyond the diffraction limit, the excitation focus is superimposed by a donut-shaped STED focus. The former excites an ensemble of markers, while the latter switches these molecules off so rapidly by stimulated emission such that they do not have time to emit a fluorescence photon.
Only in the very center of the donut, at which the STED intensity is zero, the fluorescence emission is still allowed.
The STED image is gained by scanning the fluorescent spot, whose extent depends on the STED intensity and can be theoretically decreased arbitrarily, over the sample.
isoSTED microscopy
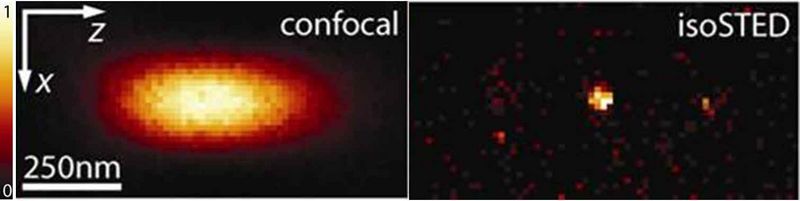
IsoSTED microscopy is a clever combination of STED and 4Pi microscopy and reduces the (cigar-shaped) confocal volume to a sphere of only 30 nm diameter. This corresponds to a reduction of the focal volume by three orders of magnitude [Schmidt et al., 2009].
The coherent use of two opposing objective lenses (4Pi configuration) renders an almost isotropic resolution in all three spatial dimensions.
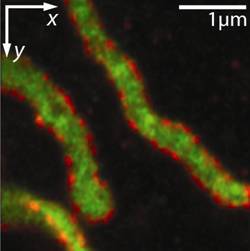
This “3D-nanosope” was the first light microscope to unravel the interior of mitochondria (the cellular power plants) in intact cells [Schmidt et al., 2008].
It was therefore possible to image the distribution of two proteins, one forming clusters on the mitochondrial membrane, the other being distributed in the interior of the organelle, with unprecedented resolution.
Further information
STED microscopy
- Website of Department of NanoBiophotonics at the MPI for biophysical chemistry: http://www.nanoscopy.de
- S. W. Hell (2007):
“Nanoskopie mit fokussiertem Licht”
Physik Journal 6 (12), 47 – 53 - S. W. Hell (2008):
“Microscopy and its focal switch”
Nature Meth. 6 (1), 24 – 32, Perspective, Special Feature, see Method of the year 2008 - S. W. Hell (2009):
“Far-Field Optical Nanoscopy”
In: Single Molecule Spectroscopy in Chemistry, Physics and Biology. Springer (Berlin, Germany), 365 – 398
isoSTED microscopy
- R. Schmidt, C. A. Wurm, S. Jakobs, J. Engelhardt, A. Egner, S. W. Hell (2008):
“Spherical nanosized focal spot unravels the interior of cells”
Nature Meth. 5 (6), 539 – 544 - R. Schmidt, C. A. Wurm, A. Punge, A. Egner, S. Jakobs, S. W. Hell (2009):
“Mitochondrial Cristae Revealed with Focused Light”
Nano Lett. 9 (6), 2508-2510 - S. W. Hell, R. Schmidt, A. Egner (2009):
“Diffraction-unlimited three-dimensional optical nanoscopy with opposing lenses”
Nature Photonics 3, 381 – 387
SMS microscopy
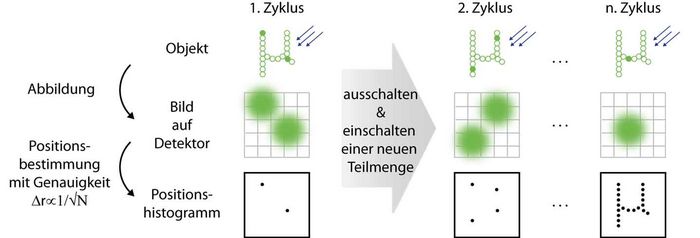
High resolution fluorescence microscopy uses the properties of fluorescent markers to overcome the diffraction limit. In contrast to ensemble-based methods (e.g. STED microscopy ), molecules are manipulated individually in SMS (single marker switching) microscopy. In this scheme, single markers are randomly selected from a large number of markers being in a dark state and transferred to a bright, detectable state by a stochastic on-switching process. The position of such a single molecule is calculated from its diffraction-limited fluorescence image which is separated spatially and temporally from the spots of other molecules. Here, the localization precision is better than the diffraction limit and scales with √N where N is the number of detected photons.
After the molecule is transferred to a dark state, this process of switching on, reading out and switching off of randomly selected markers is repeated a sufficient number of times; the histogram of all collected marker positions represents the final super-resolved SMS image.
Principle of SMS microscopy. Left: Only few markers in the sample reside in their bright state so that their diffraction-limited spots do not overlap on the detector. Their localized positions are registered in a histogram. Middle: After the markers have been read out and switched off in the first cycle, new markers are switched on and localized. Right: These cycles are repeated until sufficiently many positions have been registered to reconstruct the object
4Pi-SMS microscopy
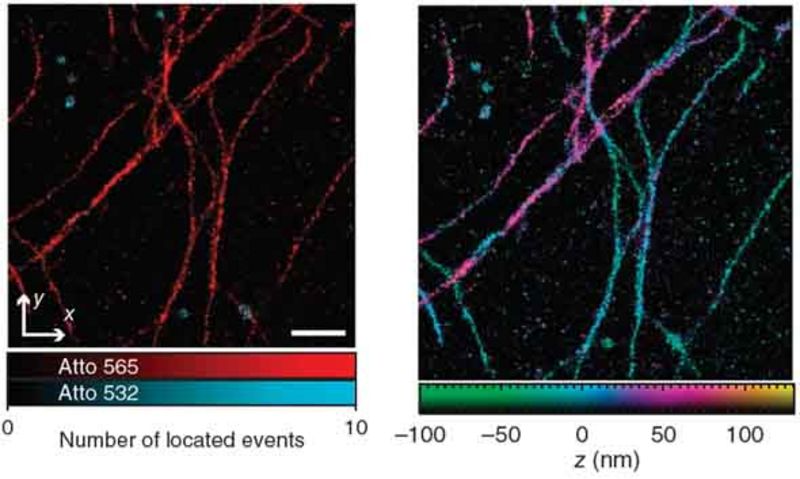
In the simplest case, an SMS image is a histogram of sufficiently many marker positions, each being calculated from the diffraction-limited fluorescence image of a single emitter. Here, the localization in the dimension along the optic axis poses a challenge. The 4Pi-SMS microscope solves this problem by using two opposing objective lenses coherently for fluorescence detection [Aquino et al., 2011]. The analysis of at least three interference patterns for each marker, each exhibiting a different relative phase, allows precise axial localization.
The 4Pi-SMS microscope can localize markers with <10 nm precision in three dimensions in an extended layer of 650 nm thickness. It can also distinguish between different species of markers, e.g. markers of different color.
Further information
SMS microscopy
- S. W. Hell:
“Microscopy and its focal switch”
Nature Meth. 6 (1), 24 – 32, Perspective, Special Feature, See Method of the year 2008 - A. Egner, C. Geisler, C. von Middendorff, H. Bock, D. Wenzel, R. Medda, M. Andresen, A. C. Stiel, S. Jakobs, C. Eggeling, A. Schönle, S. W. Hell:
“Fluorescence nanoscopy in whole cells by asynchronous localization of photoswitching emitters”
Biophys. J. 93, 3285 – 3290, 2007 - C. Geisler, A. Schönle, C. von Middendorf, H. Bock, C. Eggeling, A. Egner, S. W. Hell:
“Resolution of Lambda /10 in fluorescence microscopy using fast single molecule photo-switching”
4Pi-SMS microscopy
- D. Aquino, A. Schönle, C. Geisler, C. v. Middendorff, C. A. Wurm, Y. Okamura, T. Lang, S. W. Hell, A. Egner:
“Two-color nanoscopy of three-dimensional volumes by 4Pi detection of stochastically switched fluorophores”, Nature Methods, 8 (4), 353 – 359, 2011 - S. W. Hell, A. Egner:
“Diffraction-unlimited three-dimensional optical nanoscopy with opposing lenses”, Nature Photonics 3, 381 – 387, 2009 - C. v. Middendorff, A. Egner, C. Geisler, S. W. Hell, A. Schönle:
“Isotropic 3D Nanoscopy based on single emitter switching”
Opt. Expr. 16 (25), 20774-20788, 2008
Bioluminescence imaging
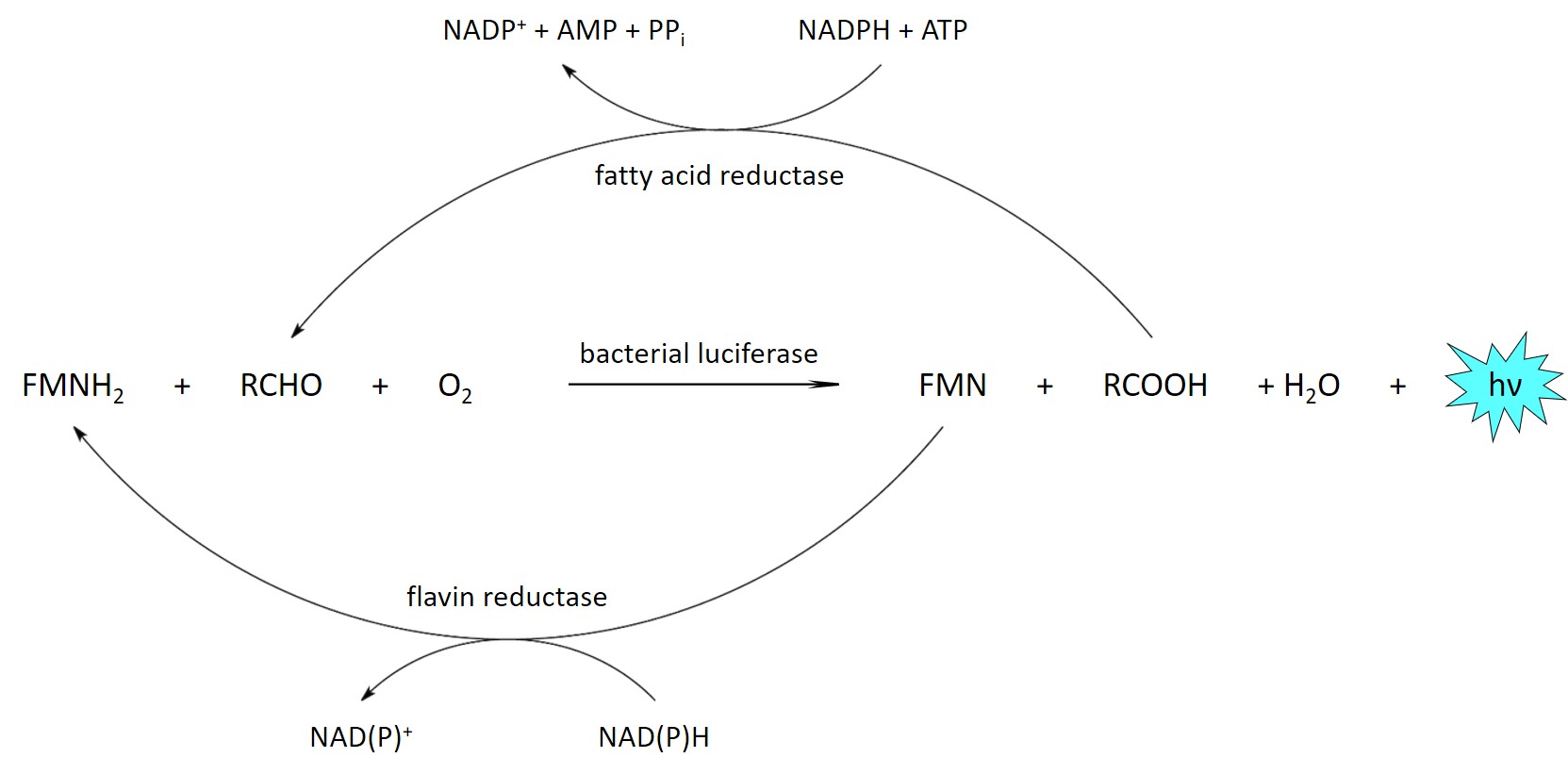
Bioluminescence light is generated in the cell by the oxidation of an organic molecule (the luciferin), forming in a product in an electronically excited state which then emits a photon. This reaction is catalyzed by an enzyme (the luciferase).
The emitted light can be recorded with a camera to obtain an image of the sample. While the luciferase is produced by the cell, the luciferin, which is consumed during the reaction, is usually added externally.
Bacterial bioluminescence system
In order to maintain continuous light emission over long periods of time, the luciferin can instead be produced by the cells themselves. This requires additional enzymes, which are not yet fully known for most bioluminescence systems, so that it is not possible to transfer the corresponding metabolic pathways to other cell types or organisms. One exception is the system from bioluminescent bacteria, which comprises several enzymes in addition to bacterial luciferase for the production of the two substrates from common cell metabolites.
Bioluminescence in bacteria
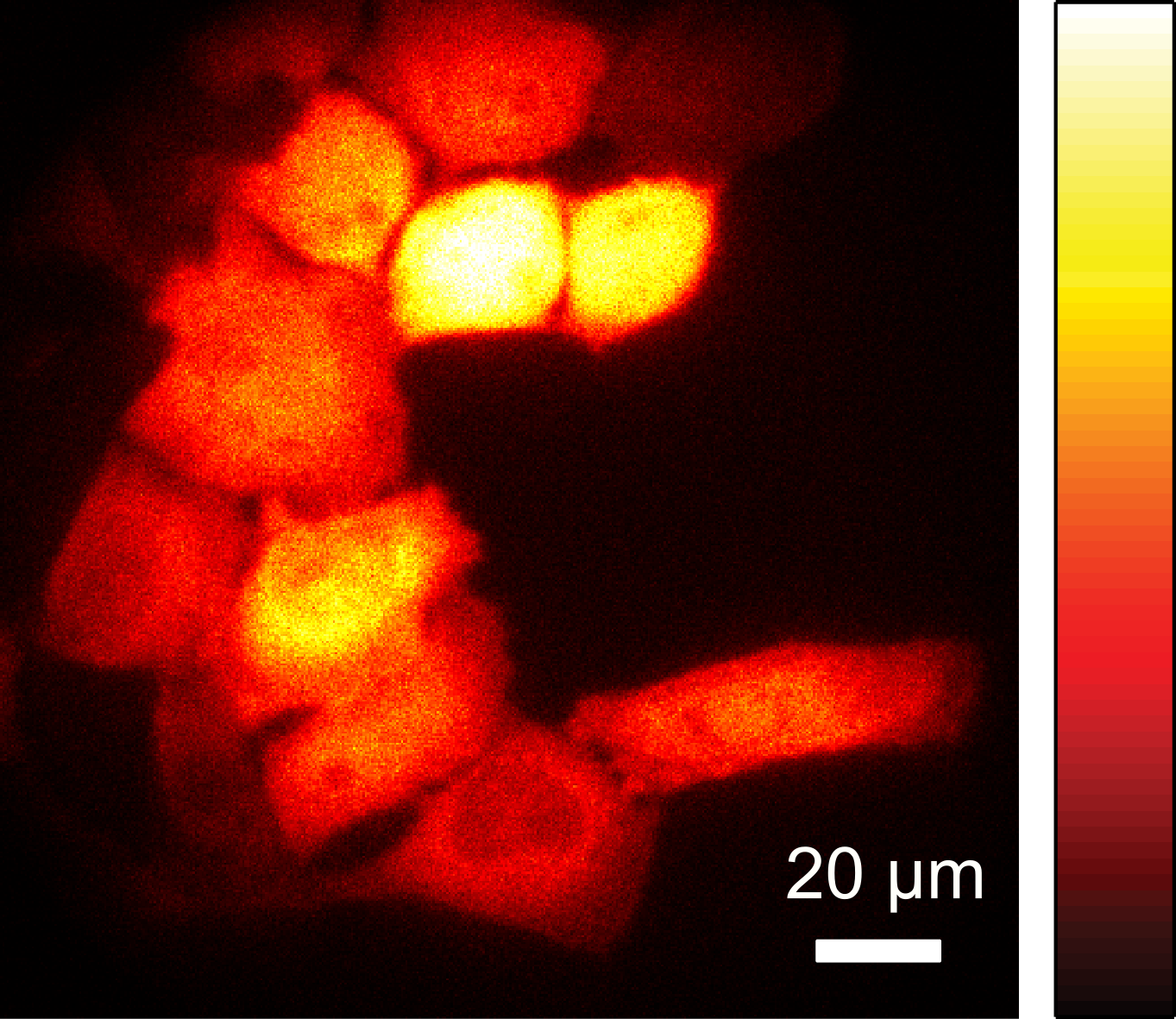
The corresponding enzymes from bioluminescent bacteria can be introduced into other bacteria where they also produce light and can be used for cellular imaging. As the brightness is comparatively low, improved variants of the enzymes have been generated which result in a significantly increased light emission in bacteria such as Escherichia coli.
This enables the observation of individual cells by bioluminescence microscopy. In addition, bacteria can also be imaged in large sample areas, for example to track their growth and spread in food over time.
Bioluminescence in mammalian cell lines
In addition to bacteria, the enzymes of the bacterial bioluminescence system can also be introduced into other cell types such as human cells to enable light emission without the addition of external substrates.
Using different approaches, the brightness of the system in mammalian cells could also be greatly increased so that individual cells can be observed with the aid of a bioluminescence microscope.
Bioluminescent organisms
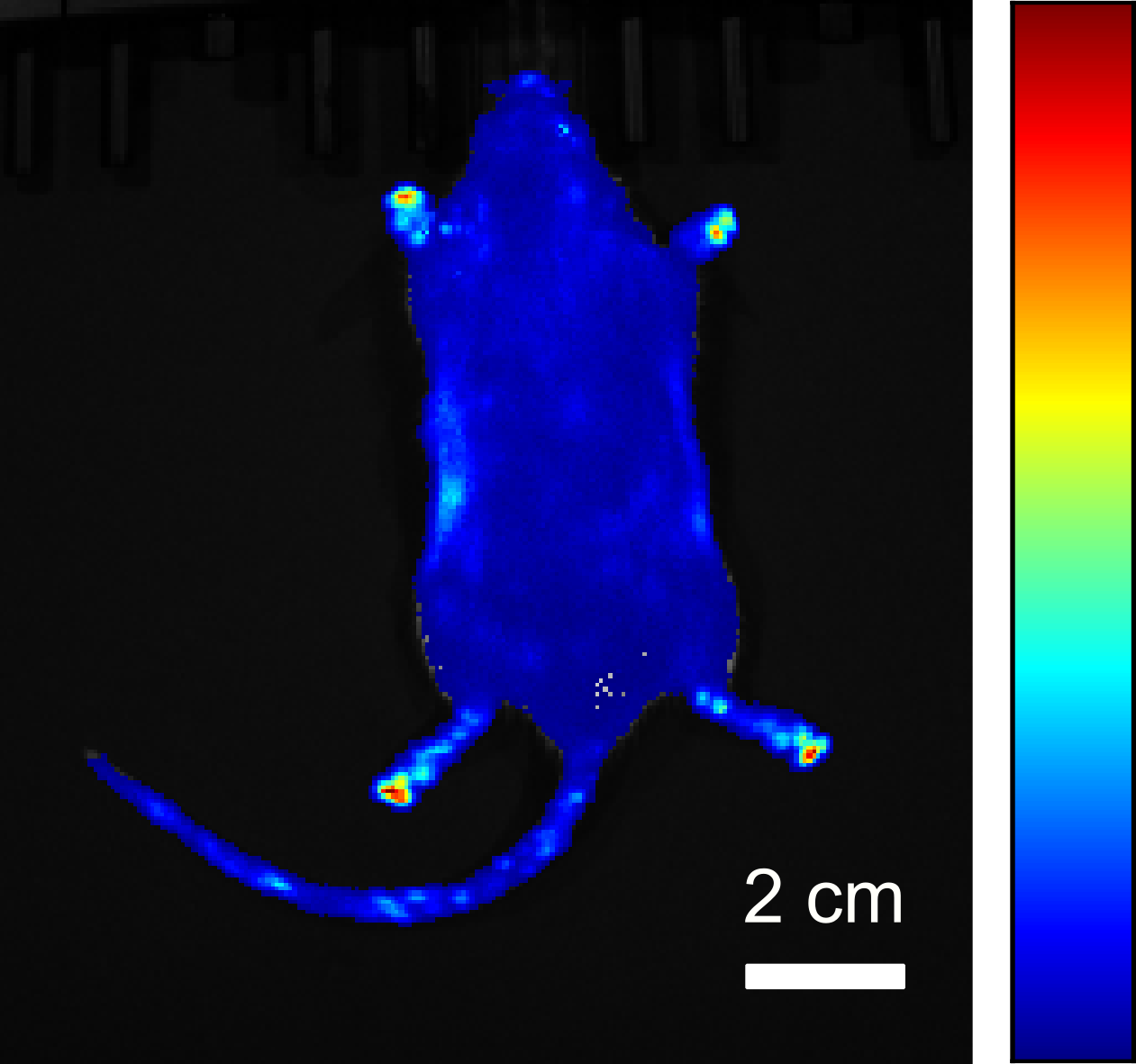
In addition to mammalian cells cultured in vitro, the bacterial bioluminescence system can also be used to establish bioluminescent properties in organisms. In this way, mouse models could be developed that emit light during their entire life span and thus enable long-term bioluminescence measurements to be carried out.
Further information
- A. Kiszka, C. Dullin, H. Steffens, T. Koenen, E. Rothermel, F. Alves, C. Gregor (2025):
“Autonomous bioluminescence emission from transgenic mice”
Science Advances 11, eads0463
- T. Brinker, C. Gregor (2024):
“Increased autonomous bioluminescence emission from mammalian cells by enhanced cofactor synthesis”
Chemosensors 12, 223
- C. Gregor (2022):
“Generation of bright autobioluminescent bacteria by chromosomal integration of the improved lux operon ilux2”
Sci. Rep. 12, 19039
- C. Gregor, J. K. Pape, K. C. Gwosch, T. Gilat, S. J. Sahl, S. W. Hell (2019):
“Autonomous bioluminescence imaging of single mammalian cells with the bacterial bioluminescence system”
Proc. Natl. Acad. Sci. U. S. A. 116, 26491 – 26496
- C. Gregor (2018):
C. “Autonome Lichtproduktion in Bakterien mit optimiertem ilux-Operon”
BIOspektrum 24, 697 – 699
- C. Gregor, K. C. Gwosch, S. J. Sahl, S. W. Hell (2018):
“Strongly enhanced bacterial bioluminescence with the ilux operon for single-cell imaging”
Proc. Natl. Acad. Sci. U. S. A. 115, 962 – 967
Products and services
Contract research Nanoscopy
- Looking for solutions for a light microscopy problem?
- Do need you a service that converts your research budget to customer-oriented and reliable solutions according to your wishes
- Do you quickly need a prototype that fits your requirements?
Our team can help you with its extensive experience, expertise and technical capabilities in the field of optical nanoscopy.
Typical tasks:
- Advice on metrological issues
- Preparation of feasibility studies
- Development of appropriate labeling strategy
- Determination of the optimum nanoscopy method for the application
- Conduct local research campaigns
- Provision of equipment
- Development of problem- and customer-oriented measurement methods
- Prototype development
Our team is ready for any new challenge.
Contact us!
We look forward to developing tailored nanoscopy and microscopy solutions for your problems.
Contact person:
Head of department
Apl. Prof. Dr. Alexander Egner
Dr. Claudia Geisler
“Optical Nanoscopy”
Tel.: +49(0)551/5035-35
Fax: +49(0)551/5035-99
alexander.egner@ifnano.de
claudia.geisler@ifnano.de
Contact person:
MBExC Junior Fellow
Dr. Carola Gregor
“Optical Nanoscopy”
Tel.: +49(0)551/5035-62
Fax: +49(0)551/5035-99
carola.gregor@ifnano.de
Staff members
Head of department
E-Mail: alexander.egner@ifnano.de
Tel.: +49 551 5035-35
Head of department
E-Mail: claudia.geisler@ifnano.de
Tel.: +49 551 5035-65
MBExC Junior Fellow
E-Mail: carola.gregor@ifnano.de
Tel.: +49 551 5035-62
Publications
Publications
- C. V. Srambickal, H. M. Esmaeeli, J. Piguet, L. Reinkensmeier, R. Siegmund, A. Agostinho, M. Bates, A. Egner, J. Widengren:
Near-infrared MINFLUX imaging enabled by suppression of fluorophore blinking, Science Advances 11(49), eadw3149 (2025) - L. Reinkensmeier, R. Siegmund, M. Bates, G. Stibenz, P. Trabs, S. Popien, I. Rimke, S. Heuke, A. Egner:
Novel Laser Technology Enables 10x Faster SRS Imaging and Rapid Tuning in Biological Samples, bioRxiv, doi: 10.1101/2025.01.31.635524 (2025) - J. I. Gallea, O. Nevskyi, Z. Kaźmierczak, I. Gligonov, T. Chen, P. Miernikiewicz, A. M. Chizhik, L. Reinkensmeier, K. Dąbrowska, M. Bates, J. Enderlein:
Super-Resolution Goes Viral: T4 Virus Particles as Versatile 3D-Bio-NanoRulers, Advanced Materials 37(12), 2403365 (2025) - I. Rimke, L. Reinkensmeier, R. Siegmund, G. Stibenz, P. Trabs, S. Popien, C. Canela, X. Gao, W. Min, A. Egner:
Fast and ultrasensitive multispectral SRS imaging with new light source, in European Conferences on Biomedical Optics 2025, Technical Digest Series (Optica Publishing Group), paper W3A.29 (2025)
Conference contributions
- N. Ayiben, R. Siegmund, A. Egner:
Background Noise and PSF Characterization for high reflecting sample in PS-SS-OCT Using the Movement of Mirror on Reference Arm, European Conference on Biomedical Optics 2025, München (06.2025) - I. Rimke, L. Reinkensmeier, R. Siegmund, G. Stibenz, P. Trabs, S. Popien, C. Canela, X. Gao, W. Min, A. Egner:
Fast and ultrasensitive multispectral SRS imaging with new light source, European Conference on Biomedical Optics 2025, München (06.2025) - J. Zhang, P. Jain, C. Geisler, S. Nagorny, T. Weingartz, A. Eitzeroth, A. Egner, C. Rembe:
1D Line-Scan Imaging of Gold Nanoparticles beyond Diffraction Limit with White Light via Absorbance Modulation Nanoscopy, Optical Measurement Systems for Industrial Inspection XIV, part of SPIE Optical Metrology, München (06.2025) - A. Egner: Pushing the Limits: Innovations in Super-Resolution Fluorescence Microscopy, Göttingen Expansion Microscopy Forum 2025, Göttingen (09.2025)
Publicationen
- C. Gregor, F. Grimm, J. Rehman, C. A. Wurm, A. Egner:
Click chemistry with cell-permeable fluorophores expands the choice of bioorthogonal markers for two-color live-cell STED nanoscopy Cells 13, 683 (2024) - T. Brinker, C. Gregor:
Increased autonomous bioluminescence emission from mammalian cells by enhanced cofactor synthesis, Chemosensors 12, 223 (2024) - I. Gallea, O. Nevskyi, Z. Kaźmierczak, T. Chen, P. Miernikiewicz, A. Chizhik, K. Dąbrowska, M. Bates, J. Enderlein:
Super-resolution going viral: T4 virus particles as perfect nature-designed 3D-Bio-NanoRulers, bioRxiv, doi: 10.1101/2024.04.04.588072 (2024)
Publications
- O. Laitenberger, T. Aspelmeier, T. Staudt, C. Geisler, A. Munk, A. Egner:
Towards Unbiased Fluorophore Counting in Superresolution Fluorescence Microscopy, Nanomaterials 13(3), 459 (2023) - P. Jain, C. Geisler, D. Leitz, V. Udachin, S. Nagorny, T. Weingartz, J. Adams, A. Schmidt, C. Rembe, A. Egner:
Super-resolution Reflection Microscopy via Absorbance Modulation, ACS Nanoscience AU 3 (5), 375-380 (2023) - S.V. Schweighofer, D.C. Jans, Jan Keller-Findeisen, A. Folmeg, M. Bates, S. Jakobs:
Endogenous BAX and BAK form mosaic rings of variable size and composition on apoptotic mitochondria (Preprint), bioRxiv (2023), https://doi.org/10.1101/2023.08.18.553869 - S. S. Stoldt, M. Barbot, T.D. Carney, F. Lange, M. Bates, P. Bou Dib, H.R. Shcherbata, M. Meinecke, D. Riedel, S. Dennerlein, P. Rehling, S. Jakobs:
The Drosophila MIC10 orthologue has a propensity to polymerize into cristae-shaping filaments (Preprint), bioRxiv (2023), https://doi.org/10.1101/2023.04.17.537183 - Antolovic, A. Egner, B. Henriques-Normark, I. Rimke, A. Schönle, J. Widengren:
Near-IR Nanoscopy and Vibrational Microscopy, Imaging & Microscopy 2023 (1), 19-21 (2023)
Conference constributions
- M. Bates:
4Pi-STORM visualization of nuclear pore complex architecture, EMBO Workshop: In situ structural biology: from cryo-EM to multi-scale modelling, EMBL Heidelberg, Heidelberg (02.2023) - M. Bates, L. Reinkensmeier, A. Egner:
Rapid Optimized Drift Correction for Single Molecule Localization Microscopy, Focus on Microscopy 2023, Porto, Portugal (04.2023) - L. Reinkensmeier:
COMET – Cost-function Optimized Maximal overlap drift EsTimation, Single Molecule Localization Microscopy Symposium SMLMS 2023, Wien, Österreich (08.2023) - A. Egner:
Gaining Deeper Insights into the Nanoworld: Surpassing the Limits of Diffraction by Innovative Light Microscopy Technology, Schnelltest-Tagung, Göttingen (09.2023) - M. Bates:
Photo-Switchable Organic Dyes: the Photophysics of SMLM, MIFOBIO Workshop: Functional Microscopy for Biology, Presqui’ile de Giens, France (11.2023) - M. Bates:
Exploring protein complex architecture with STORM microscopy, Cambridge Microscopy Meeting 2023, University of Cambridge
Publications
- Kratz, C. Geisler, A. Egner:
ISM-assisted tomographic STED microscopy, Optics Express 30(2), 939 – 956 (2022) - Bates, J. Keller-Findeisen, A. Przybylski, A. Hüper, T. Stephan, P. Ilgen, A. R. Cereceda Delgado, E. D‘Este, A. Egner, S. Jakobs, S. J. Sahl, S. W. Hell:
Optimal precision and accuracy in 4Pi-STORM using dynamic spline PSF models, Nature Methods 19(5), 603 – 612 (2022) - Wegner, H. Steffens, C. Gregor, F. Wolf, K. I. Willig:
Environmental enrichment enhances patterning and remodeling of synaptic nanoarchitecture as revealed by STED nanoscopy, eLife 11, e73603 (2022) - Gregor:
Generation of bright autobioluminescent bacteria by chromosomal integration of the improved lux operon ilux2, Scientific Reports 12, 19039 (2022) - Gregor, F. Grimm, J. Rehmann, C. A. Wurm, A. Egner:
Two-color live-cell STED nanoscopy by click labeling with cell-permeable fluorophores, BioRxiv, 2022, https://doi.org/10.1101/2022.09.11.507450Tomographic STED microscopy, Biomedical Optics Express 11(6), 3139 – 3163 (2020)
Conference constributions
- M. Bates:
Nanometer-scale 3D fluorescence imaging with 4Pi-STORM, Microscopy Club Lecture – Online (03.2022) - M. Bates, J. Keller-Findeisen, A. Przybylski, A. Hüper, T. Stephan, P. Ilgen, A.R. Cereceda Delgado, E. D’Este, A. Egner, S. Jakobs, S.J. Sahl, S.W. Hell:
Optimal Precision and Accuracy in 4Pi-STORM using Dynamic Spline PSF Models, Focus on Microscopy 2022 – Online (04.2022) - C. Gregor:
Autonomous bioluminescence microscopy of bacterial and mammalian cells, 21st International Symposium on Bioluminescence and Chemiluminescence and XIX International Symposium on Luminescence Spectrometry, Gijón, Spanien (06.2022) - M. Bates:
4Pi-STORM studies of Nuclear Pore Complex architecture, Single Molecule Localization Microscopy Symposium 2022, Paris (08.2022) (invited) - D. Ghosh, C. Geisler, A. Egner:
Photon-stream-based aberration correction for STED microscopy, DPG Tagung Regensburg 2022, Regensburg (09.2022)
Book Contributions
- C. Gregor:
Imaging of Autonomous Bioluminescence Emission from Single Mammalian Cells, In: Bioluminescence – Methods and Protocols Volume 1, Sung-Bae Kim (eds.), Methods in Molecular Biology 2524, Springer US (2022), ISBN: 978-1-0716-2452-4 (Hbk); 978-1-0716-2453-1 (pdf)
Publications
- K. Soliman, F. Grimm, C. A. Wurm, A. Egner:
Predicting the membrane permeability of organic fluorescent probes by the deep neural network based lipophilicity descriptor DeepFl‑LogP, Scientific Reports 11(1), 6991 (2021) - R. Siegmund, F. Werner, S. Jakobs, C. Geisler, A. Egner:
isoSTED microscopy with water-immersion lenses and background reduction, Biophysical Journal 120, 1 – 12 (2021) - W. Wegner, H. Steffens, C. Gregor, F. Wolf, K.I. Willig:
Environmental enrichment enhances patterning and remodeling of synaptic nanoarchitecture as revealed by STED nanoscopy, Elife 11, e73603 (2022)
Conference constributions
- J. Kratz, C. Geisler, A. Egner:
ISM-Assisted Tomographic STED Microscopy Allows for Sample-Gentle Super-Resolution Imaging,
65th Biophysical Society Annual Meeting 2021, virtual (02.2021) - J. Kratz, C. Geisler, A. Egner:
ISM-Assisted Tomographic STED Microscopy Allows for Sample-Gentle Super-Resolution Imaging,
Focus on Microscopy 2021, virtual (03.2021) - P. Jain, V. Udachin, S. Nagorny, C. Geisler, J. Adams, A. Schmidt, C. Rembe, A. Egner:
High resolution reflection microscopy via absorbance modulation, Microscience Microscopy Congress 2021, virtual, (07.2021) - C. Gregor:
Autonomous bioluminescence imaging of single cells, 16th Annual Meeting of the European Society for Molecular Imaging – EMIM 2021, Göttingen (08.2021) - C. Gregor:
Autonomous bioluminescence microscopy of bacterial and mammalian cells, 21st International Symposium on Bioluminescence and Chemiluminescence and XIX International Symposium on Luminescence Spectrometry, Gijón, Spanien (06.2022) - Autonomous bioluminescence imaging of single cells, 16th Annual Meeting of the European Society for Molecular Imaging – EMIM 2021, Göttingen (08.2021)
Publications
- T. Staudt, T. Aspelmeier, O. Laitenberger, C. Geisler, A. Egner, A. Munk:
Statistical Molecule Counting in Super-Resolution Fluorescence Microscopy: Towards Quantitative Nanoscopy, Statistical Sciences 35 (1), 92 – 111 (2020) - B. Vinçon, C. Geisler, A. Egner:
Pixel hopping enables fast STED nanoscopy at low light dose, Optics Express 28 (4), 4516 – 4528 (2020) - J.-R. Krüger, J. Keller-Findeisen, C. Geisler, A. Egner:
Tomographic STED microscopy, Biomedical Optics Express 11(6), 3139 – 3163 (2020)
Book contributions
- A. Egner, C. Geisler, R. Siegmund:
STED nanoscopy, In: Nanoscale Photonic Imaging, T. Salditt et al. (eds.), Topics in Applied Physics 134, Springer International Publishing (2020)
Conference constributions
- B. Vinçon, C. Geisler, A. Egner:
Ultrafast Switchable Depletion Patterns for tomoSTED Microscopy Generated by Conical Diffraction, Focus on Microscopy 2019, London, Großbritannien (04.2019) - A. Egner:
isoSTED microscopy in living cells, International Symposium on Nanoscale Photonic Imaging, Venedig, Italien (03.2019) - A. Egner:
Quantitative Microscopy, Cluster of Excellence (EXC 171) & DFG Research Center (FZT 103) – CNMPB Final Symposium, Göttingen (08.2019) - C. Geisler:
Optical Nanoscopy: Imaging beyond the diffraction limit, CINSat Autum Colloquium 2019, Universität Kassel, Kassel (10.2019) - C. Geisler, R. Kowarsch, P. Jain, C. Rembe, A. Egner:
Hochauflösende Reflexionsmikroskopie mittels Absorptionsmodulation, – Photonic Net – Forum MikroskopieTrends ’19, Göttingen (12.2019)
Publications
- R. Kowarsch, C. Geisler, A. Egner, C. Rembe:
Superresolution reflection microscopy via absorbance modulation: A theoretical study,
Opt. Ex. 26 (5) (2018)
- B. Roubinet, M. Bischoff, S. Nizamov, S. Yan, C. Geisler, S. Stoldt, G. Y. Mitronova, V. N. Belov,
M. L. Bossi, S. W. Hell:
Photoactivatable Rhodamine Spiroamides and Diazoketones Decorated with “Universal Hydrophilizer” or Hydroxyl Groups, J. Org. Chem. 83, 12 (2018)
- E. Neubert, D. Meyer, F. Rocca, G. Günay, J. Grandke, A. Kwaczala-Tessmann, S. Senger-Sander, C. Geisler, A. Egner, M. Schön, L. Erpenbeck, S. Kruss:
Chromatin swelling drives neutrophil extracellular trap release, Nature Communications 9,1:3767 (2018)
Conference constributions
- A. Egner:
Lichtblicke in die Nanowelt – Neue Methoden der Lichtmikroskopie überlisten die Beugung, Saturday Morning Lecture, Universität Hannover (01.2018) - K. Soliman, F. Rocca, R. Siegmund, C. Geisler, A. Egner:
STED nanoscopy: imaging beyond the diffraction limit of light, 23rd Dubai International Pharmaceuticals and Technologies Conference and Exhibition DUPHAT 2018, Dubai (02.2018)J.-R. Krüger, B. Vinçon, C. Geisler, A. Egner:
Overcoming the resolution barrier by STED microscopy, SPIE Optics + Photonics, Nanoscience + Engineering, Nanoimaging and Nanospectroscopy VI, San Diego, CA, USA (08.2018) - D. Köhne, C. Geisler, P. Simon, A. Egner:Novel STED-assisted nanostructuring method for subtractive sub-diffraction writing, SPIE Optics + Photonics, Nanoscience + Engineering, Nanoimaging and Nanospectroscopy VI, San Diego, CA, USA (08.2018)
- R. Kowarsch, C. Geisler, A. Egner, C. Rembe:
Modeling superresolution reflection microscopy via absorbance modulation, SPIE Optics + Photonics, Nanoscience + Engineering, Nanoimaging and Nanospectroscopy VI, San Diego, CA, USA (08.2018) - A. Egner:
Subdiffraction-resolution fluorescence imaging, SFB 1286 Symposium ‘Advanced microscopy & biomedicine meets NanoSIMS’, Göttingen (10.2018)
Conference constributions
- R. Siegmund, C. Geisler, A. Egner:
IsoSTED microscopy in living cells, SFB 755 Winterschool 2017,
Hirschegg Kleinwalsertal, Österreich, (02.2017)
- J.-R. Krüger, J. Keller-Findeisen, C. Geisler, A. Egner:
Tomographic STED microsopy, CNMPB-Retreat,
Bad Sooden-Allendorf (02.2017)
- F. Rocca, N. Mazkereth, J. Krüger, C. Geisler, A. Egner, Z. Fishelson:
Two-Color STED microscope for the analysis of stress-induced interaction between
mitochondrial chaperone mortalin and the plasma membrane attack complex,
Focus on Microscopy 2017, Bordeaux, France (04.2017)
- J. Krüger, J. Keller-Findeisen, B. Vincon, C. Geisler, A. Egner:
Tomographic STED microscopy, Focus on Microscopy 2017,
Bordeaux, France (04.2017)
- H. Mittelstädt, C. Geisler, A. Egner:
Single marker switching microscope with isotropic resolution over large axial range,
SPIE Optics + Photonics, Nanoscience + Engineering, Nanoimaging and Nanospectroscopy V,
San Diego, CA, USA (08.2017) - J.-R. Krüger, J. Keller-Findeisen, C. Geisler, A. Egner:
Tomographic STED microscopy,
SPIE Optics + Photonics, Nanoscience + Engineering,
Nanoimaging and Nanospectroscopy V, San Diego, CA, USA (08.2017) - A. Egner:
Optical nanoscopy: Decoding the building blocks of life beyond the diffraction barrier, XXXI. AHMT Symposium 2017, Clausthal-Zellerfeld (09.2017) - A. Egner:
Optical nanoscopy and statistics: Towards the optimum resolution, Statistics Meets Friends – from biophysics to inverse problems and back, Göttingen (11.2017) - A. Egner:
Tomographic STED Microscopy, Forum MikroskopieTrends ´17, Göttingen (12.2017)
Publications
- A. Gucek, J. Jorgacevski, P. Singh, C. Geisler, M. Lisjak, N. Vardjan, M. Kreft, A. Egner, R. Zorec:
“Dominant negative SNARE peptides stabilize the fusion pore in a narrow, release-unproductive state”, Cell. Mol. Life Sci. (2016).
- S. J. Sahl, F. Balzarotti, J. Keller-Findeisen, M. Leutenegger, V. Westphal, A. Egner, F. Lavoie-Cardinal, A. Chmyrov, T. Grotjohann, S. Jakobs:
„Comment on “extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics”. Science 352 (6285), 527-527, (2016)
- N. Mazkereth, F. Rocca, J. Schubert, C. Geisler, Y. Hillman, A. Egner, Z. Fishelson:
„Complement triggers relocation of Mortalin/GRP75 frommitochondria to the plasma membrane”, Immunobiology, (2016)
Conference constributions
- H. Grefe, C. Geisler, A. Egner:
Novel 3D single marker switching microscope with isotropic resolution over large axial range,
SPIE Photonics West 2016, Single Molecule Spectroscopy and Superresolution Imaging IX,
San Francisco, USA (02.2016)
- A. Egner:
New developments in optical nanoscopy, International Symposium 2016 “Biological Dynamics from Microscopic to Mesoscopic Scales”, Grimma (02.2016)
- R. Siegmund, C. Geisler, A. Egner:
isoSTED microscopy in living cells, International Symposium 2016 “Biological Dynamics from Microscopic to Mesoscopic Scales”, Grimma (02.2016)
- H. Mittelstädt, C. Geisler, A. Egner:
Single Marker Switching Microscope with Isotropic Resolution over Large Axial Range, Focus on Microscopy 2016, Taipei, Taiwan (03.2016)
- J. Schubert, J. Keller-Findeisen, C. Geisler, B. Vincon, A. Egner:
Low-intensity STED microscope with increased image brightness and uncompromised resolution,
80. Jahrestagung der DPG und DPG-Frühjahrstagung, Regensburg (03.2016)
- A. Egner:
Super-resolved fluorescence microscopy: Principle and applications of STED microscopy, BioImaging Symposium 2016, München (04.2016)
- D. Köhne, C. Geisler, P. Simon, A. Egner:
Principles and applications of optical switching assisted imaging and structuring schemes, International Conference on Physics 2016, New Orleans, USA (06.2016)
- A. Egner, J. Schubert, C. Geisler, B. Vincon:
Low-intensity STED microscope with increased image brightness and uncompromised resolution,
SPIE Nanoscience + Engineering, Nanoimaging and Nanospectroscopy IV,
San Diego, USA (08.2016)
- A. Egner:
Super-resolved fluorescence microscopy: Principle and applications of STED microscopy,
623th Wilhelm and Else Heraeus-Seminar on “Cellular Dynamics”, Bad Honnef (09.2016)
- R. Siegmund, F. Rocca, C. Geisler, A. Egner:
isoSTED microscopy in living cells, International Symposium 2016 “Biological Dynamics from Microscopic to Mesoscopic Scales”, Grimma (02.2016)
- A. Egner, A. Munk:
Statistical reconstruction methods for time varying nanoscale imaging problems,
International Symposium 2016 “Biological Dynamics from Microscopic to Mesoscopic Scales”,
Grimma (02.2016)
- B. Vinçon, J.-R. Krüger, C. Geisler, A. Egner:
Ultrafast switchable depletion patterns for STED microscopy generated by conical diffraction, 623th Wilhelm and Else Heraeus-Seminar on “Cellular Dynamics”, Bad Honnef (09.2016)
- F. Rocca, N. Mazkereth, J.-R. Krüger, C. Geisler, A. Egner, Z. Fishelson:
Complement triggers relocation of Mortalin/GRP75 from mitochondria to the plasma membrane, 623th Wilhelm and Else Heraeus-Seminar on “Cellular Dynamics”, Bad Honnef (09.2016)
Publications
- A. Hartmann, S. Huckemann, J. Dannemann, O. Laitenberger, C. Geisler, A. Egner, A. Munk:
Drift Estimation in sparse sequential dynamic imaging: with application to nanoscale fluorescence microscopy, J. R. Stat. Soc. B, doi: 10.1111/rssb.12128 (2015)
- T. Aspelmeier, A. Egner, A. Munk:
Modern Statistical Challenges in High-Resolution Fluorescence Microscopy, Annu. Rev. Stat. Appl. 2, 163–202 (2015)
Conference constributions
- R. Siegmund, C. Geisler, F. Rocca, C. A. Wurm, S. Jakobs, A. Egner:
Three Dimensional IsoSTED Microscopy In Living Cells, Focus on Microscopy 2015, Göttingen (03.2015)
- O. Laitenberger, A. Hartmann, C. Geisler, A. Munk, A. Egner:
Statistical Drift Estimation And Correction Methods For Single Marker Switching Based Microscopy, Focus on Microscopy 2015, Göttingen (03.2015)
- H. Grefe, C. Geisler, A. Egner:
Novel 3D Single Marker Switching Microscope With Isotropic Resolution Over Large Axial Range, Focus on Microscopy 2015, Göttingen (03.2015)
- A. Egner, C. Geisler, H. Grefe, J. Schubert:
Developments in fluorescence nanoscopy, Optics + Photonics, Nanoimaging and Nanospectroscopy III, San Diego (08.2015)
- R. Siegmund, C. Geisler, A. Egner:
isoSTED microscopy in living cells, Optics + Photonics, Nanoimaging and Nanospectroscopy III, San Diego (08.2015)
- A. Egner:
Super-Resolved Fluorescence Microscopy: Principle and Applications of STED Microscopy, IMB seminar, Tromsø (10.2015)
- A. Egner:
Angewandte Optik – abbilden, analysieren und strukturieren mit Licht, Bier & Brezeln, Göttingen (11.2015)
- J.-R. Schubert, C. Geisler, A. Egner:
Low-Intensity STED Microscope With Increased Image Brightness And Uncompromised Resolution, Focus on Microscopy 2015, Göttingen (03.2015)
Publications
- A. Hartmann, S. Huckemann, J. Dannemann, O. Laitenberger, C. Geisler, A. Egner, A. Munk:
Drift Estimation in sparse sequential dynamic imaging: with application to nanoscale fluorescence microscopy arxiv.org, 1403.1389, (2014) - V. N. Belov, G. Y. Mitronova, M. L. Bossi, V. P. Boyarskiy, E. Hebisch, C. Geisler, K. Kolmakov, C. A. Wurm, K. I. Willig, S. W. Hell:
Masked Rhodamine Dyes of Five Principal Colors Revealed by Photolysis of a 2-Diazo-1-Indanone Caging Group: Synthesis, Photophysics, and Light Microscopy Applications, Chem. Eur. J. 20, 13162-13173 (2014)
Conference constributions
- A. Egner:
Overcoming the resolution barrier by STED microscopy Symposium on Advanced Microscopy Techniques in Life Sciences, Homburg (04.2014)
- A. Egner:
Three‐dimensional optical nanoscopy with opposing lenses SPIE Optics and Photonics, San Diego (08.2014)
- A. Egner:
Three‐dimensional optical nanoscopy with opposing lenses 18th International Microscopy Congress, Prague (09.2014)
- A. Egner:
Optical Nanoscopy meets Statistiks: Decoding the Building Blocks of Life Eröffnungssymposium des Felix‐Bernstein Institut für Mathematische Statistik in den Biowissenschaften, Göttingen, (10.2014)
Conference constributions
- A. Egner:”Three-dimensional optical nanoscopy with opposing lenses”Symposium on
„Super Resolution Imaging“, Göttingen (2013) - C. Geisler, R. Schmidt, D. Aquino, S. W. Hell, A. Egner:
“Developments in Fluorescence Nanoscopy”
SPIE Optics + Photonics, San Diego (2013) - C. Geisler, T. Hotz, A. Schönle, S. W. Hell, A. Munk, A. Egner:
“Drift estimation for single marker switching based imaging schemes”
SPIE Optics + Photonics, San Diego (2013) - A. Egner:
“Superresolution Microscopy”
Fourth Annual Conference “Superresolution Microscopy”, Ljubljana (2013) - C. Geisler, J. Jorgačevski, M. Kreft, R. Zorec, A. Egner:
“Two-color STED microscopy”
Fourth Annual Conference “Superresolution Microscopy”, Ljubljana (2013) - A. Egner:
“Overcoming the resolution barrier by STED microscopy”
Visualizing the invisible in cell biology, Symposium on super-resolution microscopy, Prague (2013)
Publications
- C. Geisler, T. Hotz, A. Schönle, S. W. Hell, A. Munk, A. Egner:
“Drift estimation for single marker switching based imaging schemes”
Opt. Express 20, 7274 (2012) - F. Opazo, M. Levy, M. Byrom, C. Schäfer, C. Geisler, T. W. Groemer, A. D. Ellington, S. O. Rizzoli:
“Aptamers as potential tools for superresolution microscopy”
Nat. Meth. 9, 938 (2012) - A. C. Meyer, D. Khimich, A. Egner, T. Moser:
“Super-resolution optical microscopy of the organ of Corti.
Investigations on the fine structure of the inner hair cell afferent synapse by the 4Pi and STED techniques.”
HNO 60, 707 (2012) - G. Whelan, E. Kreidl, G. Wutz, A. Egner, J. M. Peters, G. Eichele:
“Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin.”
EMBO J. 31, 71 (2012)
Conference constributions
- A. Egner:
Superresolution Microscopy, Jahrestagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie.
Dresden (03.2012) - J. Ihlemann, C. Geisler, R. Schmidt, D. Aquino, S. W. Hell, A. Egner:
Developments in Fluorescence Nanoscopy. 14th International Congress of Histochemistry and Cytochemistry, Kyoto (08.2012)
Publications
- D. Aquino, A. Schönle, C. Geisler, C. v. Middendorff, C. A. Wurm, Y. Okamura, T. Lang, S. W. Hell, A. Egner:
“Two-color nanoscopy of three-dimensional volumes by 4Pi detection of stochastically switched fluorophores”. Nature Methods, 8 (4), 353 – 359, (2011) - Y. Okamura, R. Schmidt, I. Raschke, M. Hintze, S. Takeoka, A. Egner, T. Lang:
“A Few Immobilized Thrombins Are Sufficient for Platelet Spreading”
Biophysical Journal, Volume 100, Issue 8, (2011) - Y. Hua, R. Sinha, C. Thiel, R. Schmidt, J. Huve, H. Martens, S. W. Hell, A. Egner, J. Klingauf:
“A readily retrievable pool of synaptic vesicles”, Nature Neuroscience, doi:10.1038/nn.2838, (2011) - C. A. Wurm, D. Neumann, M. A. Lauterbach, B. Harke, A. Egner, S. W. Hell, S. Jakobs:
“Nanoscale distribution of mitochondrial import receptor Tom20 is adjusted to cellular conditions and exhibits an inner-cellular gradient”
PNAS, doi:10.1073/pnas.1107553108, (2011) - K. Kumar, L. Isa, A. Egner, R. Schmidt, M. Textor, E. Reimhult:
“Formation of Nanopore-Spanning Lipid Bilayers through Liposome Fusion!”Langmuir, 27, 10920-10928, (2011) - C. K. Ullal, S. Primpke, R. Schmidt, U. Böhm, A. Egner, P. Vana, S. W. Hell:
“Flexible Microdomain Specific Staining of Block Copolymers for 3D Optical Nanoscopy”
Macromolecules, 44, 7508-7510, (2011)
Conference constributions
- Egner A.:
Diffraction-unlimited 3D optical microscopy with opposing lenses, Photonics West, San Francisco, (01.2011) - Egner A.:
isoSTED & 4Pi-SMS microscopy, Focus on Microscopy, Konstanz, (04.2011) - Egner A.:
Optical switching and time-sequential coherent detection of markers through opposing lenses enables multicolor 3D-nanoscopy with 10-nm resolution of large intracellular volumes, European Conference on Biomedical Optics, München, (05.2011)
Publications
- G. Vicidomini, R. Schmidt, A. Egner, S. W. Hell, A. Schönle:
“Automatic deconvolution in 4Pi-microscopy with variable phase”
Opt. Exp. 18 (8), 10154 – 10167, (2010) - C. A. Wurm, D. Neumann, R. Schmidt, A. Egner and S. Jakobs:
“Sample Preparation for STED Microscopy”
In: D.B. Papkovsky (ed.), Live Cell Imaging, Methods in Molecular Biology 591, DOI 10.1007/978-1-60761-404-3 11, © Humana Press, a part of Springer Science+Business Media,
LLC (2010) - T. Frank, M. A. Rutherford, N. Strenzke, A. Neef, T. Pangrsic, D. Khimich, A. Fejtova, E. D. Gundelfinger, M. C. Liberman, B. Harke, K. E. Bryan, A. Lee, A. Egner, D. Riedel, T. Moser:
“Bassoon and the Synaptic Ribbon Organize Ca2+ Channels and Vesicles to Add Release Sites and Promote Refilling”
Neuron, 68, 724-738, (2010)
Publications
- S. W. Hell, R. Schmidt, A. Egner:
“Diffraction-unlimited three-dimensional optical nanoscopy with opposing lenses” (Nature Photonics 3, 381 – 387), (2009) - A. C. Meyer, T. Frank, D. Khimich, G. Hoch, D. Riedel, N. M. Chapochnikov, Y. M. Yarin, B. Harke, S. W. Hell,
A. Egner, T. Moser:
“Tuning of synapse number, structure and function in the cochlea”, Nature Neuroscience 12 (4), 444-453, (2009) - R. Schmidt, C. A. Wurm, A. Punge, A. Egner, S. Jakobs, S. W. Hell:
“Mitochondrial Cristae Revealed with Focused Light”, Nano Lett. 9 (6), 2508-2510, (2009) - C. K. Ullal, R. Schmidt, S. W. Hell, A. Egner:
“Block Copolymer Nanostructures Mapped by Far-Field Optics”, Nano Lett. 9 (6), 2497-2500 (2009) - G. Perinetti, T. Müller, A. Spaar, R. Polishchuk, A. Luini, A. Egner:
“Correlation of 4Pi and Electron Microscopy to Study Transport Through Single Golgi Stacks in Living Cells with Super Resolution”, Traffic 10, 379-391, (2009)
- R. Schmidt, C. A. Wurm, S. Jakobs, J. Engelhardt, A. Egner, S. W. Hell:
“Spherical nanosized focal spot unravels the interior of cells”
Nature Meth. 5 (6), 539 – 544 (2008) - M. Andresen, A. C. Stiel, J. Fölling, D. Wenzel, A. Schönle, A. Egner, C. Eggeling, S. W. Hell, S. Jakobs:
“Photoswitchable fluorescent proteins enable monochromatic multilabel imaging and dual color fluorescence nanoscopy”
Nature Biotech. 26 (9), 1035-1040 (2008) - A. C. Stiel, M. Andresen, H. Bock, M. Hilbert, J. Schilde, A. Schönle, C. Eggeling, A. Egner, S. W. Hell, S. Jakobs:
“Generation of Monomeric Reversibly Switchable Red Fluorescent Proteins for Far-Field Fluorescence Nanoscopy”
Biophys. J. 95, 2989-2997 (2008) - M. Bossi, J. Fölling, V. N. Belov, V. P. Boyarskiy, R. Medda, A. Egner, C. Eggeling, A. Schönle, S. W. Hell:
“Multicolor Far-Field Fluorescence Nanoscopy through Isolated Detection of Distinct Molecular Species”
Nano Letters 8 (8), 2463 – 2468 (2008) - C. v. Middendorff, A. Egner, C. Geisler, S. W. Hell, A. Schönle:
“Isotropic 3D Nanoscopy based on single emitter switching”
Opt. Expr. 16 (25), 20774-20788 (2008) - I. Testa, A. Schönle, C. v. Middendorff, C. Geisler, R. Medda, C. A. Wurm, A. C. Stiel, S. Jakobs, M. Bossi, C. Eggeling, S. W. Hell, A. Egner:
“Nanoscale separation of molecular species based on their rotational mobility”
Opt. Expr. 16 (25), 21093 – 21104 (2008) - J. Fölling, V. Belov, D. Riedel, A. Schönle, A. Egner, C. Eggeling, M. Bossi, S. W. Hell:
“Fluorescence Nanoscopy with Optical Sectioning by Two-Photon Induced Molecular Switching using Continuous-Wave Lasers”
ChemPhysChem 9, 321 – 326 (2008)
- A. Egner, C. Geisler, C. von Middendorff, H. Bock, D. Wenzel, R. Medda, M. Andresen, A. C. Stiel, S. Jakobs, C. Eggeling, A. Schönle, S. W. Hell:
“Fluorescence nanoscopy in whole cells by asynchronous localization of photoswitching emitters”, Biophys. J. 93, 3285 – 3290 (2007) - J. Fölling, V. Belov, R. Kunetsky, R. Medda, A. Schönle, A. Egner, C. Eggeling, M. Bossi, S. W. Hell:
“Photochromic Rhodamines provide Nanoscopy with Optical Sectioning”
Angew. Chem. Int. Ed. 46, 6266 – 6270 (2007) - H. Bock, C. Geisler, C. Wurm, S. Jakobs, A. Schönle, A. Egner, S.W. Hell, C. Eggeling:
“Two-color far-field fluorescence nanoscopy based on photoswitching emitters”, Appl. Phys. B 88, 161-165 (2007) - C. Geisler, A. Schönle, C. von Middendorf, H. Bock, C. Eggeling, A. Egner, S.W. Hell:
“Resolution of Lambda /10 in fluorescence microscopy using fast single molecule photo-switching”, Appl. Phys. A 88, 223-226 (2007) - C. Eggeling, M. Hilbert, H. Bock, C. Ringemann, M. Hofmann, A. C. Stiel, M. Andresen, S. Jakobs, A. Egner, A. Schönle, S. W. Hell:
“Reversible Photoswitching Enables Single-Molecule Fluorescence Fluctuation Spectroscopy at High Molecular Concentration”
Micr. Res. Techn. 70, 1003 – 1009 (2007)
- A. Egner, S. W. Hell:
“Fluorescence microscopy with super-resolved optical sections”
Trends Cell Biol. 15 (4): 207 – 215 (2005) - A. Egner, S. Verrier, A. Goroshkov, H.-D. Söling and S. W. Hell:
“4Pi-microscopy of the Golgi apparatus in live mammalian cells”
J. Struct. Biol. 147(1): 70-76 (2004) - S. Jakobs, N. Martini, A. C. Schauss, A. Egner, B. Westermann, S. W. Hell:
“Spatial and temporal dynamics of budding yeast mitochondria lacking division component Fis1p”
J. Cell Sci. 116: 2005-2014 (2003) - A. Egner, S. Jakobs and S. W. Hell:
“Fast 100-nm resolution 3D-microscope reveals structural plasticity of mitochondria in live yeast”
Proc. Natl. Acad. Sci. USA 99: 3370-3375 (2002) - A. Egner, V. Andresen, S. W. Hell:
“Comparison of the axial resolution of practical Nipkow-disk confocal fluorescence microscopy with that of multifocal multiphoton microscopy: theory and experiment”
J. Microsc. 206: 24-32 (2003) - V. Andresen, A. Egner, S. W. Hell:
“Time-multiplexed multifocal multiphoton microscope”
Opt. Lett. 26(2): 75-77 (2003) - A. Egner and S. W. Hell:
“Time multiplexing and parallelization in multifocal multiphoton microscopy”
J. Opt. Soc. Am. A 17(7): 1192-1201 (2003) - T. Klar, S. Jakobs, M. Dyba, A. Egner, S. W. Hell:
“Fluorescence microscopy with diffraction resolution limit broken by stimulated emission”
PNAS 97(15): 8206-8210 (2000) - A. Egner, S. W. Hell:
“Equivalence of the Huygens-Fresnel and Debye approach for the calculation of high aperture point-spread-functions in the presence of refractive index mismatch”
J. Microsc. 193(3): 244-249 (1999) - A. Egner, M. Schrader, S. W. Hell:
“Refractive index mismatch induced intensity and phase variations in fluorescence confocal, multiphoton and 4Pi-microscopy”
Opt. Commun. 153: 211-217 (1998)